

Ellume recalls COVID-19 home test for potential false positive SARS-CoV-2 test results. The performance of certain COVID-19 home-testing kits seems to have deteriorated after the omicron variant emerged, according to a new study published Thursday by the British Medical Journal. DxTerity SARS-CoV-2 RT-PCR CE test EUA summary. LabCorp COVID-19 RT-PCR test EUA summary.ĭxTerity. BinaxNOW COVID-19 Ag card home test kit: procedure card. Lucira check-it COVID-19 test kit.Ībbott. Saliva testing is accurate for early-stage and presymptomatic COVID-19. Factors that influence the reported sensitivity of rapid antigen testing for SARS-CoV-2. Interim guidance for SARS-CoV-2 testing in non-healthcare workplaces. Coronavirus disease 2019 testing basics.Ĭenters for Disease Control and Prevention. At-home OTC COVID-19 diagnostic tests.Ĭenters for Disease Control and Prevention. Emergency use authorization of COVID-19 tests: Independent assessment of the FDA's response.įood and Drug Administration. Please be aware that we do not take any responsibility for your accessing such information that may not comply with any legal process, regulation, registration, or usage in the country of your origin.Food and Drug Administration. Additionally, this website contains information on products that is targeted to many different audiences and could contain product details or information otherwise not accessible or valid in your country. If you do not agree to these terms, do not use the website. By accessing, browsing and/or using this website, you acknowledge that you have read, understood, and agree to be bound by Quidel’s Website Terms and Conditions, Privacy Policy and to comply with all applicable laws and regulations. No use of any Quidel trademark, trade name, or trade dress in this site may be made without the prior written authorization of Quidel, except to identify the product or services of the company. Unless otherwise specified, all product and service names appearing in this Internet site are trademarks owned by or licensed to Quidel, its subsidiaries or affiliates. The Vault saliva test kits are polymerase chain reaction (PCR) tests, molecular tests that detect the viruss genetic material. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.Ĭopyright ©2021 Quidel Corporation. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C.
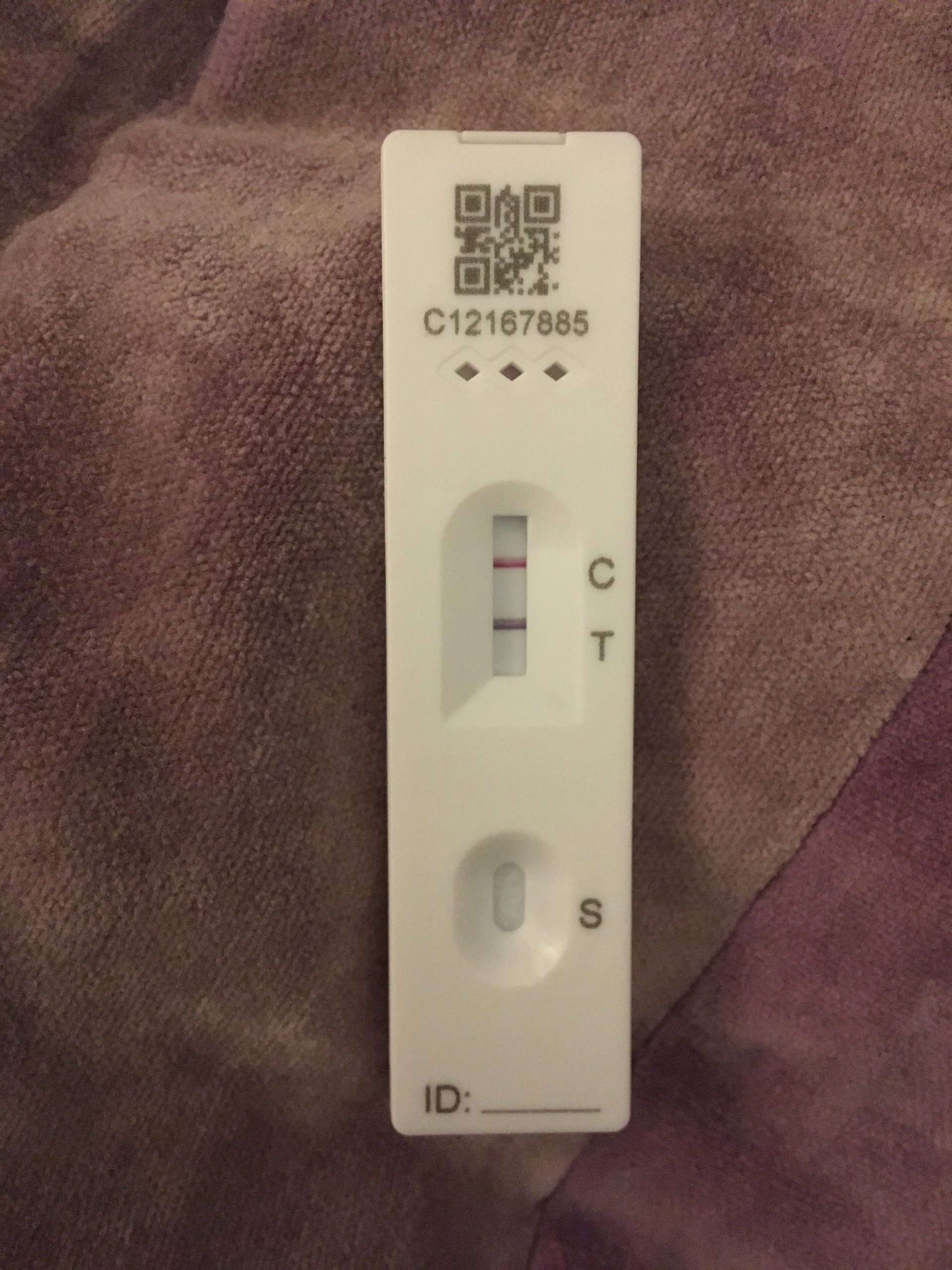
This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. *In the USA, this product has not been FDA cleared or approved but has been authorized by FDA under an Emergency Use Authorization. This test is authorized for nonprescription home use with self-collected (unobserved) direct anterior nasal (NS) swab specimens from individuals aged 14 years and older or with adult-collected anterior NS samples from individuals aged 2 years or older.
#AT HOME COVOD TEST SERIAL#
Serial testing should be performed in individuals with negative results at least twice over three days (with 48 hours between tests) for symptomatic individuals and three times over five days (with at least 48 hours between tests) for asymptomatic individuals. The QuickVue At-Home OTC COVID-19 Test is intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19.


 0 kommentar(er)
0 kommentar(er)
